WARNING, MAY CAUSE SIDE-EFFECTS: NANOPARTICLES IN THE ENVIRONMENT
By: Carolyn Wilke
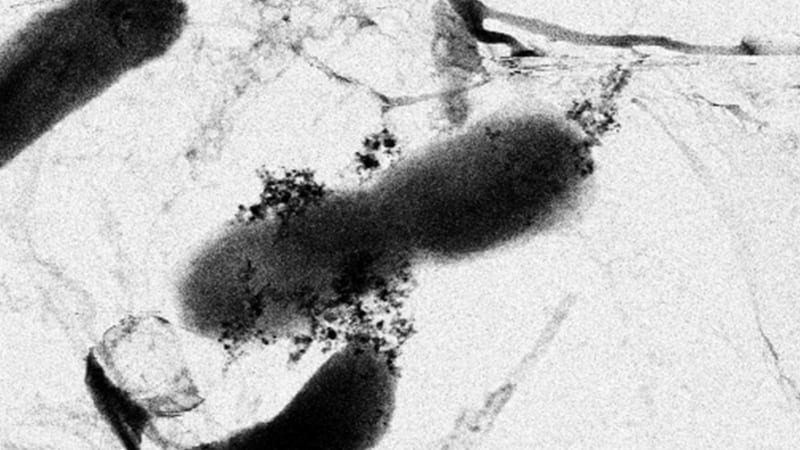
E Coli bacteria surrounded by a cluster of titanium oxide and silver nanoparticles.
Image copyright C Wilke/Gray Research Group, all rights reserved. Used by permission of the author.
If you watch television at all, you’ve seen advertisements suggesting that some new medicine is the best remedy for a particular disease. In stark contrast to a montage of agile women practicing yoga in a field of flowers and a pain-free granddad tossing a football to an adoring child, a soothing voice issues a warning: this drug has potential side effects and there is a litany of medicines that shouldn’t be used at the same time. This is because our bodies may react very differently to combinations of drugs than to the prescription alone, with possibly dangerous consequences.
As with medicines in our bodies, chemicals in the environment act and interact differently when multiple are present. For plants, insects, or microbes, chemical contaminants may be more or less toxic in combination than they are alone.
I study a new group of contaminants to our environment: nanoparticles. In a recent video, I explain how these minuscule pieces of matter get into the environment from consumer products. We use lots of everyday items—clothing, personal care products, food, and more—that contain nanoparticles. Nanoparticles can enter the environment at any point of the product’s lifecycle: when they are manufactured, when we use them, or after we dispose of the products that contain them. For example, nanoparticles are used in many different personal care products (like makeup and sunscreen) and wash off of us when we go for a swim at the beach or take a bath.
Once in the environment, nanoparticles like these can react with other chemical substances, including other nanoparticles that have found their way there. When bacteria encounter multiple types of nanoparticles several different interactions may occur. The nanoparticles may behave independently — so that their toxicity together would be a straightforward addition of their individual effects — but multiple nanoparticles could also drastically amplify or lower the combined toxicity compared to each nanoparticle alone.
Our research team studies the effects of combined nanoparticle toxicity to bacteria, many of which perform important and necessary tasks in the world’s ecosystems. My work is to understand how specific nanoparticles can be more or less toxic to bacteria when multiple types appear together in messy environmental systems.
Recently, my coworkers and I published a paper in Environmental Science & Technology studying toxicity changes when titanium dioxide and silver nanoparticles are together. Titanium dioxide is incredibly common in consumer goods like paints, sunscreen, and even food, while silver nanoparticles are used in many household products because of their antimicrobial powers. These are two of the most commonly used types of nanoparticles that could potentially meet in a natural body of water, like a river or lake.
We tested the toxicity of silver and titanium dioxide nanoparticles to E. coli bacteria in the dark. Normally, silver nanoparticles are highly toxic to bacteria, but we found that the presence of titanium dioxide nanoparticles reduces silver’s lethal effects.
So how does it work? When silver nanoparticles dissolve, they release highly toxic silver ions. Titanium dioxide nanoparticles can capture these toxic silver ions through a process called adsorption, in which ions are bound to the particle’s surface. This means fewer silver ions floating around in the water and a less toxic environment for the bacteria. But titanium dioxide has a limit to how much silver it can hold on to. As a result, we found titanium dioxide’s ability to lower the toxicity of silver only applies to relatively low concentrations of silver nanoparticles.
Results like these demonstrate the toxicity of any particular nanoparticle is context specific: toxicity is determined by both the nanoparticle’s properties and the ways the environment – that includes other contaminants and nanoparticles — transform it.
